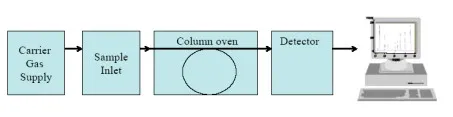
Gas Chromatograph
Gas chromatograph HP6890A gas chromatography is the separation of a gaseous mixture into its individual elements. In principle, every component can be identified through gas chromatography, assuming it is sufficiently volatile and thermally stable. The institute’s gas chromatograph is equipped with two capillary columns. The first column is an HP-PLOT-Q (length of the column: 30 m, diameter: 0.53 mm, film thickness: 40 µm). This column was developed specifically for the separation of hydrocarbons (CH4, ethylene, propylene, C1 to C3 isomers) of CO2, components containing sulfur (H2S, COS) and polar solvents (methanol, acetone, alcohols, ...).
The second column is a molecular sieve column HP-PLOT-5A Molesieve (length of the column: 30 m, diameter: 0.53 mm, film thickness: 25 µm). It separates the permanent gases such as H2, O2, CH4 and CO.
To begin the gas analysis, these two columns are set up in a row. Since the molecular sieve column would absorb CO2 permanently, the columns are set up parallel to each other, shortly before the CO2 leaves the first column.
The gas chromatograph consists of the feed for the carrier gas, the feed for the sample, the columns, which are integrated into an oven, the detector, and a data acquisition and analysis device (PC).
Diagram 1: Schematic depiction of the gas chromatograph.
The carrier gas (helium) sets the moving phase in motion, which takes up the tested gas, and streams it along a stationary, solid phase. The individual components of the tested gas are adsorbed at the surface of the stationary phase. The length of the adsorption process varies depending on the component, which means that the residence time within the column for a specific component is characteristic of that component. After the column, the separated components reach the detector.
The detector used in the gas chromatograph at the institute provides a voltage signal in the µV range.
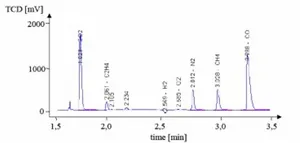
Diagram 2: Gas chromatogram
The result of the gas analysis is easily presentable through a chromatogram. The position of each peak is characteristic of a component, as it is dependent on the residence time of the component in the column. The area under the peak, the integral of the signal, is a measure for the mole fraction of the material in the tested gas. In order to identify the component, and to quantify its share, a calibration of the measuring device is required.
The analysis program at the institute could be optimized so that it would take only three and a half minutes for a gas analysis to be completed. For the application of a gas chromatograph as an online gas analysis method, however, this is too long. Further disadvantages of gas chromatography are that the tested gas may not contain any steam, as this could lead to the deterioration of the stationary phase in the column. The measurements could be corrupted, if remnants of a previous gas analysis still reside in the column.