Discharging
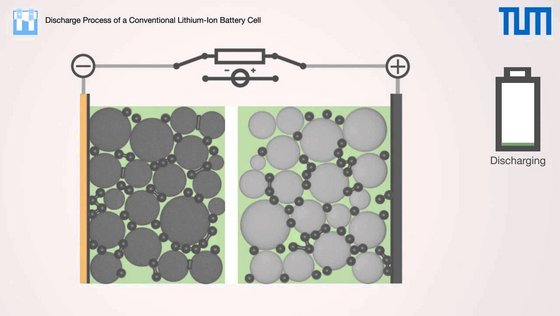
During the first stage of discharge lithium atoms oxidize by forming Li+ ions and electrons, whereas Li+ ions move to the positive electrode diffusing through the electrolyte and the separator. The electrons flow from the negative electrode to the positive on the external circuitry, where the resulting current flow can be used for an application. At the positive electrode the electrons recombine with the Li+ ions and are stored in the molecular structure of the active material.
Charging:
If an external voltage with the same polarity is applied between the current collectors, the charge process will start. The lithium atoms leave the metal oxid structure and ionize into Li+ ions under the release of an electron of each one. In analogy with the discharge process Li+ ions diffuse to the negative electrode. At the surface of the graphite particles the Li+ ions and electrons recombine with each other forming neutral lithium atoms and are reintercalated into the molecular structure of the graphite particles.